As cancers continue to rise, Rakuten Medical is at the forefront of cancer treatment innovation, showcased through its unique Alluminox platform

Over the last 25-30 years, regional competitors have replicated Japanese manufacturing processes, achieving cost advantages through cheaper labor and pushing Japan out of certain mass markets. However, Japan still maintains leadership in the medical field. For example, the country holds a 98% global market share in flexible endoscopes. Despite stiff regional competition, how have Japanese firms managed to maintain leadership in the medical field?
That is a good question. Let's discuss a few key areas where Japan leads, including technologies like endoscopes and MRI, as well as biotechnologies in general. We can categorize these areas into three brackets: firstly, Japan excels in the invention, or discovery, of new technologies. For instance, Dr. Honjo discovered the PD1/PDL1 coupling at Kyoto University. In terms of invention and discovery, Japan's strong academic institutes and research centers have played a significant role. Their strength can be attributed to higher educational standards and government investment. Japan compares well with countries like the US and European nations in this aspect.
Secondly, Japan has been successful in incubating technologies for commercialization. Some areas stand out in this regard, such as endoscopes, MRI, and other measurement and diagnostic systems. The success in these areas can be attributed partly to Japan's healthcare system, which has a strong emphasis on early detection and prevention compared to countries like the US, which focus more on cures. The combination of Japan's industrial strengths, such as semiconductors and cameras, with the incubation of inventions, has contributed to this success.
Thirdly, the commercial model of incubation plays a crucial role. While Japan excels in invention and incubation, there have been challenges in commercialization. Few companies have achieved significant success on a global scale. We are particularly focused on oncology at the moment. Japan is particularly good at discovery rather than incubation, which is seen more across the Atlantic, in the US. Nevertheless, Japan's strength lies in discovery, invention, and incubation, particularly in device technologies related to detection and diagnosis.
One of the significant criticisms of Japan’s medical sector has been its slow regulatory processes. On average, it takes about three to five years longer than Europe or America for approvals. However, since 2012, we have witnessed some improvements, a notable being the Sakigake system. From your point of view, how would you assess the Japanese regulatory system, and what are some of the improvements you would like to see going forward?
Everyone says, “Japan is slow and bureaucratic”, but my answer is “No”. Regulatory frameworks, such as the FDA in the US, PMDA in Japan, and EMA in Europe, are pretty consistent. Although some differences exist in the specific application processes, the overall framework remains the same. In terms of speed, they are pretty similar and synchronized. They keep track of each other's activities.
PMDA, as well as other regulatory agencies under the broader scope of the Japanese government, such as the Ministry of Health, Labor, and Welfare, are doing the right job for Japan. The perception of slowness primarily stems from the industry side, which fails to recognize that they are paying taxes to receive services from the Ministry of Healthcare. Regulatory approval is one of the services provided by the government to make drugs available to citizens. The agencies need sufficient data and rational justification to say yes to a technology for the benefit of Japan and its taxpayers. This rationalization is necessary. The downstream processes, such as review and approval, are well-defined and efficient.
The agencies need to be scientifically rational in their decision-making. When companies submit documents or engage with regulatory bodies, they must present a scientifically rational case. Therefore, it is more about how the industry can work together with regulatory agencies in Japan. That is my general perspective, and that's why we are working here as a medical company.
Alluminox is a technical platform used for developing new treatments for various diseases, including cancer. It combines drugs and light to induce selective cell necrosis, which may stimulate the immune system. Could you go into more detail about this Alluminox platform and what key developments and findings has this platform allowed you to discover?
The Alluminox technology is based on an invention by Dr Kobayashi and Dr Peter L Choyke of the National Cancer Institute (NCI) in the US. The invention aimed to induce cell necrosis, or selective cell death, for any targeted cell. Pre-clinical data have shown that this technology can destroy a specific cell by targeting proteins or markers present on its surface. The concept of photoimmunotherapy involves combining drugs and light to achieve selective cell killing.
At our company, we are developing this technology for several applications. The simplest use case is targeting specific proteins overexpressing in cancer cells, which may allow us to selectively kill those cells while sparing healthy cells. We are currently focusing on developing cancer treatment. If we can identify a specific protein that is unique to cancer cells, we can selectively target those cancer cells.
However, our approach goes beyond just killing targeted cancer cells. Through our preclinical research, we know we can also target other cancer-associated cells, such as immune cells (e.g., regulatory T cells) that suppress the immune system's response to cancer. By depleting these immunosuppressive cells, we hope to trigger tumor-specific immune responses. Our technology strives for a multi-approach to cancer treatment.
Our approach involves changing the target moiety, which is currently achieved using antibodies. By modifying the antibodies, we can target different types of cancer cells or cancer-associated cells. This flexibility allows us to adapt the treatment to various cancer types. For instance, we are currently developing and commercializing a drug called ASP-1929, which uses an antibody called EGFR as the targeting moiety. However, by switching to another antibody, we can target different cancer cells or associated cells. This concept of platform technology enables us to develop tailored treatments based on specific needs.
In Japan, ASP-1929 photoimmunotherapy is approved as a treatment for locally advanced or locally recurrent head and neck cancers that cannot be surgically treated. As we understand, you are currently undergoing a global phase III multi-center clinical trial with the drug in patients with recurrent head and neck squamous cell carcinoma (HNSCC), so can you tell us what are your expectations for this trial?
We are seeking to demonstrate the effectiveness and safety of ASP-1929 photoimmunotherapy as a monotherapy for recurrent HNSCC patients who have already failed at least two types of cancer therapy and hope to provide meaningful clinical outcomes for these patients who have very limited treatment options. It is a global study, so we are seeking approval in multiple locations, considering the global nature of cancer and the presence of many companies working in this field.
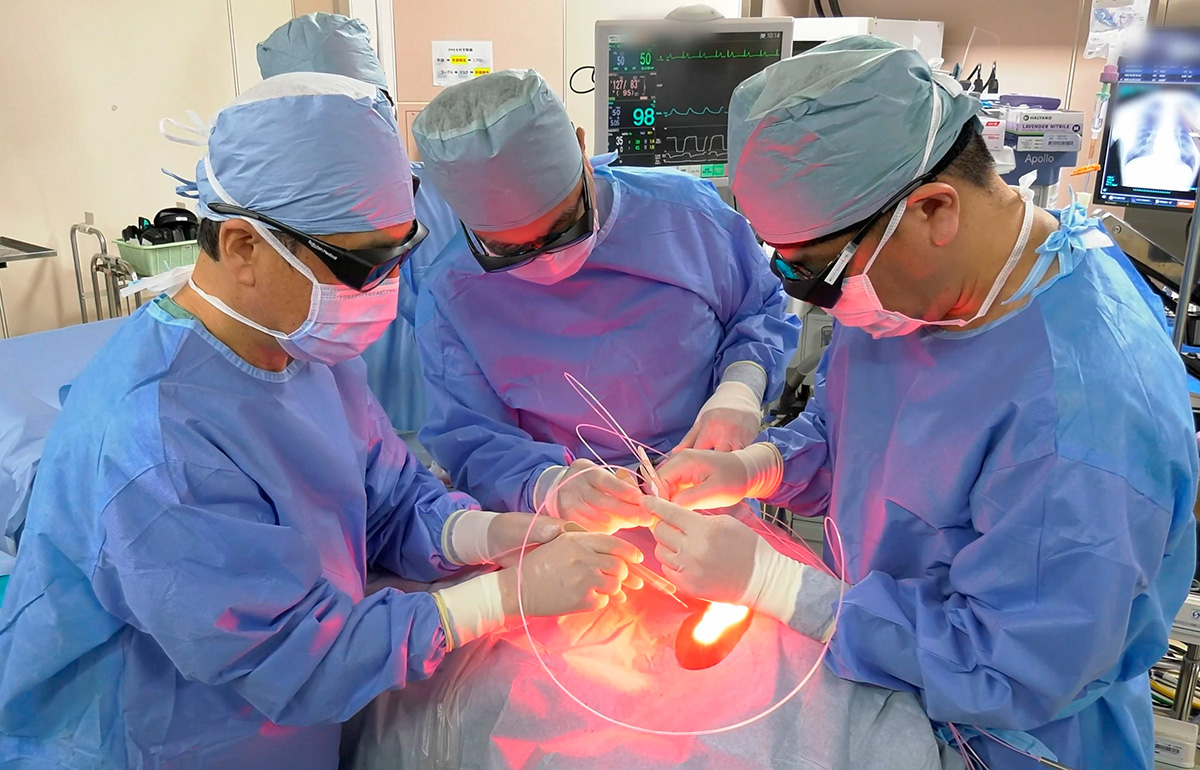
Alluminox treatment
Chemotherapy damages both cancer cells and healthy cells, resulting in various side effects such as hair loss and pain. These side effects can be very uncomfortable for patients. You have discussed photo immunotherapy as an alternative treatment. Do you believe photoimmunotherapy will eventually overtake chemotherapy, and if so, how long until we get to that point?
I would never say that our technology is absolutely the best, but I believe it will give patients more choices. Of course, we would like there to be fewer side effects, and we are aiming for that, and want to make our system one of the main choices for cancer patients. Choosing the best technology for curing cancer is crucial for our industry, ecosystem, and society. I am determined to develop our technology as a top contender in this field. Currently, there are a few conditions that need to be addressed, such as the side effects and toxicity associated with chemotherapy. While chemotherapy has undeniably saved many lives over the years, we must find ways to overcome these drawbacks. By developing our technology appropriately, we may be able to contribute to this aspect.
In addition to surgery, major treatment modalities such as chemotherapy, radiation and immunotherapy are already in use. In the short, medium, and even long terms, these modalities are likely to be combined. For instance, chemotherapy is often used in combination with radiation, and immunotherapy shows promising potential with radiation and chemotherapy. It is crucial to consider not only the toxicity and safety of these treatments but also their compatibility with other technologies. Waiting for one treatment modality to fail before utilizing another is scientifically sound, but it is unacceptable when there are promising alternatives available. Therefore, it is essential to develop a combination-friendly approach that allows patients to benefit from multiple therapies simultaneously. To achieve this, we should focus on developing technologies that offer targeted, low-dosage, and less frequent treatment options. Patients should not have to endure lengthy and frequent treatment cycles. Our technology has the potential to address these needs in the short to medium term.
Before COVID, according to the International Agency for Research on Cancer (IARC), there were 17 million new cancer cases with 9.5 million deaths. They predicted by the year 2040, it is expected that cancer cases would grow to about 27.5 million with about 16.3 million deaths. Between 2018 and 2021, cancer increased by about 4.7%. Why do you believe we are seeing an increase in cancer cases and what are some of the challenges, and opportunities this presents for Rakuten Medical?
By definition, as life expectancy increases, the chances of developing cancer also rise. This is due to advancements in our living environments. I am not implying that having more opportunities for cancer is desirable, it is just that a happy scenario where cancer is eradicated from society is not happening in the short term.
As you mentioned, early detection is crucial when our life expectancy is extended. While our primary focus is on treatment, early detection still holds significance. Cancer, by its nature, is more effectively treated in the early stages when the immune system is still active, and tumors are smaller. Early detection allows for a broader range of treatment options. When you have a longer life expectancy and detect cancer early, you have 20-30 years remaining, giving you more time to choose from different treatment options.
Naturally, it is preferable to have less invasive and less toxic treatments for early-stage disease. Therefore, a combination of early detection and treatment is essential. Advancements in endoscope technology, such as endoscopic submucosal dissection (ESD), are heading in that direction, enabling simultaneous detection and treatment. However, the challenge lies in the fact that cancer occurs at a cellular level, which is not visible to the naked eye.
Detection becomes an issue until a visible tumour or a significant number of cancer cells develop. Consequently, treatment needs to address the cellular, micro, and nano scales. This is where I am eager to contribute by developing our technology to target cellular and immune system-level early-stage treatment. This is the direction everyone is striving for, including the development of next-generation immuno-oncology drugs that go beyond PD-1 and PD-L1, focusing on tailor-made cellular-level diagnosis and treatment in conjunction. The treatment technology we are developing is needed in this domain to complement diagnostic techniques.
The second area to consider is the affordability and accessibility of cancer treatment and detection. As you mentioned, the number of cancer patients will double or even triple in the short term, and the majority of patients will reside in countries with lower income levels. Therefore, the technology should be designed to be cost-effective and user-friendly. For example, intravenous infusion is a commonly used method, but there is room for technological advancements to make it simpler and more efficient. These are the areas where we should focus our efforts. Early-stage detection combined with treatment, affordable options, and user-friendly technology should be the direction for all treatments, considering the progression of the disease.
The question for the industry is how we can apply and evolve our technology in that direction. As a developer of new technology, I am actively seeking opportunities in this domain. Other companies focusing on quality drugs may have a different approach, but they, too, are likely looking to contribute to society.
Through the Alluminox Alliance Institute program, you developed relationships with medical institutes specializing in cancer all across the world. Can you tell us more about the role that these alliances play in your business model, and how do you plan on further increasing the Overseas Alliance network that you have established so far?
Our technology, the Alluminox platform, is a combination of drug and device technology. As of today, we require operational procedures and partnerships with medical centers, beyond just physicians. These partnerships involve medical centers providing solutions, not just us offering technology. Commercialization is a separate matter. Once we have a specific set of treatment procedures combined with our technology, we can deploy it on a scalable level. However, for development purposes, we need top institutes to partner with us and provide feedback from the field to further refine and advance our technology. This iterative process is crucial.
We have already announced partnerships with several institutes in the US. However, it is important to note that our focus is not limited to the US alone. Japan also. We have significant collaborative sites and partnerships in other countries like Taiwan. The medical needs for cancer treatment vary by region. For example, the healthcare systems and timelines for early detection differ between Japan and the US. Additionally, factors like diet, environment, and healthcare infrastructure vary, resulting in different affordable pricing and treatment options. Viruses like HPV, which impact cancer growth, are also spread unevenly around the world.
Therefore, our strategy is to identify the best partners and sites in each individual territory, collaborate to provide tailored solutions for patients and receive appropriate feedback. These partnerships involve fair and scientifically rigorous discussions with medical professionals, aiming to bring the right insights to our company and foster development and evolution in line with the single aim of improving cancer treatment.
Apart from clinical and medical partnerships, we also have partnerships in other areas. One key area is devices. While we develop our own devices, there are companies specializing in detection or laser technology, for example, who possess expertise that complements ours. Our concept is to take responsibility for supplying the combined solution while broadening our technological partnerships with other companies. We aim to seek the best solutions for medical needs, regardless of the source, be it detection technology or other relevant expertise. We are open to collaboration and welcome partners who can contribute to the development of antibodies, for instance. Financial partners are also welcomed as part of our collaborative approach.

San Diego headquarters
Rakuten Medical was founded in San Diego, USA, in 2010. In addition to the United States and Japan operations, you now have locations in Taiwan, Switzerland, Holland and India. Moving forward, which regions have you identified as key for growth, and what strategies will you employ?
Our core belief is to seek excellence worldwide. While we operate in multiple countries, we do not favour one over the other. We are a global company committed to serving global patients in need of our technology. Our operations gradually expand, but we maintain specific core countries, such as the US and Japan, for strategic reasons. The US is crucial for proper biotech operations due to regulatory frameworks, business models, and access to centers of innovation. Similarly, Japan is significant due to its regulatory openness and its position as the second-largest pharma spender, with outstanding technology and skilled physicians.
We can leverage Japan itself as an incubation hub for our technology, taking advantage of the favourable regulatory environment. We also recognize Taiwan as a strategic partner in our international strategy. Taiwan is a truly global country with top-notch talent that can compete on an international level. Due to its unique position, Taiwan has a significant number of immigrants from South Asian countries like Malaysia, while also maintaining close ties with mainland China and having good connections with Japan. Taiwan is a sizable country, not too big or too small. We are leveraging Taiwan as a valuable partner, not only for our company but also for establishing strong partnerships with other countries like India.
Speaking of India, it holds great importance for us. Approximately one-fourth of all cancer patients in India suffer from head and neck cancer, accounting for around 25% of all new cases. It is essential for us to develop technology that caters to the specific needs of head and neck cancer patients in India. We have previous experience working in India and recognize its significance in our overall strategy. It is crucial for us to address the needs of patients wherever we operate. As a global company, our operations are integrated across countries, and we do not differentiate between locations. We strive to leverage our strengths in each territory, aligning our strategies accordingly.
Imagine we come back in exactly five years, and we interview you again: what goals would you like to have the company achieve by then?
I can guarantee you that even if you come here after five years, I will be talking about the same story and the same ambition. We are committed to putting patients first, ensuring ethical practices, and staying ahead of cancer treatment trends, such as early detection and less invasive treatments. If you were to interview me again in five years, my dream would be to see more patients benefiting from our technology more appropriately. By then, we would have received approvals and deployed our technology on a larger scale. Our technology will be more advanced, also. I envision hearing positive feedback from a broader range of patients. While I do not have specific numerical targets in mind, I hope to make a significant impact and receive widespread recognition for our contributions. That is my vision here.
Japan has the potential to contribute more to the global landscape, and it should not be seen as an isolated island. As the CEO of a US company, I genuinely believe that Japan can play a crucial role in fostering innovation and improving patient outcomes globally. This is not about competition or who is better. Our industry needs to think about how we can collectively leverage Japan's strengths for the benefit of patients. We need to work together as an industry to create a positive impact on society and provide benefits not only to ourselves but also to our families and the wider community.
Interview conducted by Karune Walker & Ana Ruiz
0 COMMENTS